A new paper entitled “Hydraulic and biotic impacts on neutralisation of high-pH waters“ has just been published online and in open access on the journal Science of the Total Environment. This paper is available here.
Our research showed that cascades lower pH and alkalinity more successfully than ponds, and that configuration should be adopted, if possible, in the passive treatment of high-pH waters.
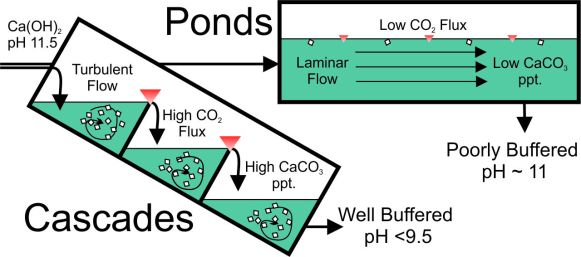
We could also observe that biofilms promote neutralisation due to CO2 from respiration. In fact, photosynthesis and respiration in biofilms induce a diurnal effect at high pH. The pH variation in biofilm colonized systems shows a diurnal cycle of 1 to 1.5 pH units due to CO2 uptake and release associated with respiration and photosynthesis. However, the hydraulic configuration has more influence on neutralisation of alkaline waters than biofilm.
The management of alkaline (pH 11-12.5) leachate is an important issue associated with the conditioning, afteruse or disposal of steel slags (an important by-product of the steel industry). Passive in-gassing of atmospheric CO2 is a low cost option for reducing alkalinity, producing calcium carbonate.
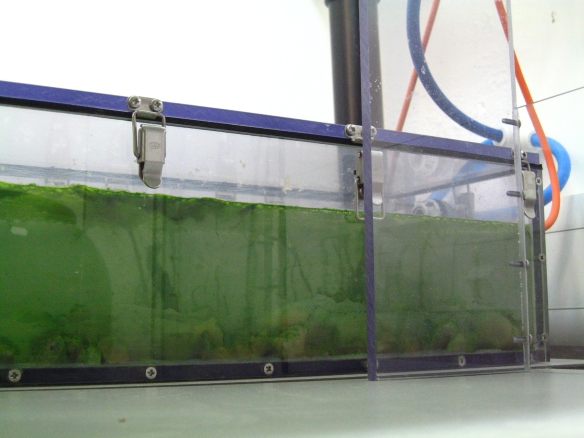
View of the biofilm colonized systems used in the experiments
 Our team has another publication entitled: “
Our team has another publication entitled: “


































