We would like to invite you to the workshop “How can policy and regulation support resource recovery from waste?” on 21st September 2017 in Durham, Kenworthy Hall at St Marys College. This workshop is organised as part of a collaborative mini-project by the Resource Recovery from Waste programme and associated researchers of the AVAnD, B3, MeteoRR and R3AW projects.
This is a one day workshop on Vanadium recovery from steel slag landfills.
At this workshop, you will gain insight into the Resource Recovery from Waste programme and the technologies developed within our projects. You will get the opportunity to share best practice in policies and regulations that enable resource recovery, while also highlighting any barriers that may exist. We will use the project findings in our work striving for positive change in government policy supporting a circular economy in the UK.
This is one of four workshops across the country. Each workshop strives to answer the question: “If we wanted to realise resource recovery in the UK, how would it be possible within our policy and regulatory context?”. We will ask for your knowledge and experience to carry out a policy analysis, identifying drivers and barriers for resource recovery in general and for specific technologies, and identify which actors could drive required changes in the policy and regulation landscape
Understanding how change in the governance of waste and resource management can be achieved is vital to promote resource recovery and increaser resource efficiency as part of the transition towards the circular economy. Based on this research, we will formulate policy recommendations for governmental bodies throughout the UK.Each workshop strives to answer the question: “If we wanted to realise resource recovery in the UK, how would it be possible within our policy and regulatory context?” We will ask for your knowledge and experience to carry out a policy analysis, identifying drivers and barriers for resource recovery in general and for specific technologies, and identify which actors could drive required changes in the policy and regulation landscape.
This workshop aims to bring together people from academia, government, and industry. Please contact Anne Velenturf A.Velenturf@leeds.ac.uk to register for the workshop. Workshop spaces are limited and will be allocated on a first come, first serve basis.


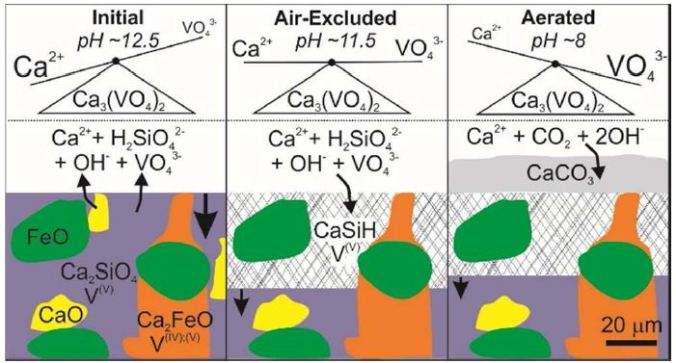



 The paper “
The paper “

 A new review paper on alkaline wastes
A new review paper on alkaline wastes 
